CTXII (短名 ),
产品名称:
Human CTXII/ C-telopeptide of Collagen alpha-1(II) chain ELISA Kit
胶原蛋白α -1(II)链的c端肽
货号:
E0686h
商标:
EIAab®
监管等级:
检测方法:
ELISA
实验类型:
Competitive
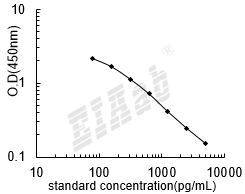
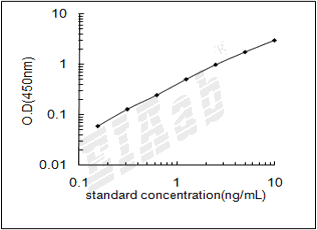
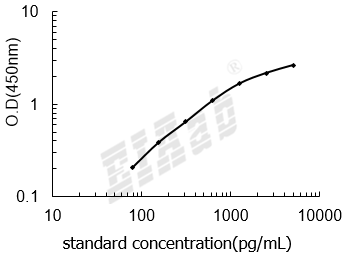
检测范围:
78-5000pg/mL
灵敏度:
32pg/mL
特异性:
Natural and recombinant human C-telopeptide of Collagen alpha-1(II) chain
样品类型:
Serum, plasma, tissue homogenates, cell culture supernates and other biological fluids
样品数据:
登录.
实验步骤:

研究领域:
-
精密度
批内差:已知浓度的3个样本在一个板子内重复检测20次,以评估批内精密度。
批内 CV: ≤4.3%
批间差:已知浓度的3个样本在不同的板子上重复测定5次,以评估测定批间精密度。
批间 CV: ≤7.5%
批内 CV: ≤4.3%
批间差:已知浓度的3个样本在不同的板子上重复测定5次,以评估测定批间精密度。
批间 CV: ≤7.5%
回收率
回收率:低、中和高浓度的分析物被掺入到血清或者血浆样本中,进行回收实验测定。
|
Sample Type |
Average(%) |
Recovery Range(%) |
|
Serum |
100 |
94-106 |
|
Plasma |
102 |
96-108 |
线性
线性:给定样本通过梯度稀释,每次稀释的测量值与理论值的比值。
|
Sample |
1:2 |
1:4 |
1:8 |
1:16 |
|
serum(n=5) |
89-97% |
102-112% |
108-116% |
106-115% |
|
EDTA plasma(n=5) |
104-114% |
110-119% |
107-117% |
95-105% |
|
heparin plasma(n=5) |
107-117%
|
104-113% |
91-100% |
99-109% |
通用注释
亚单元:
N/A
功能:
In bone physiology, the C-terminal telopeptide (or more formally, carboxy-terminal collagen crosslinks, and known by the acronym CTX) is a serum biomarker used to measure the rate of bone turnover. It can be useful in assisting clinicians to determine a patient's nonsurgical treatment response as well as evaluate a patient's risk of developing complications during healing following surgical intervention. The test used to detect the CTX marker is called the Serum CrossLaps, and it is more specific to bone resorption than any other test currently available.
Location:
N/A
该产品尚未在任何出版物中被引用。
[1].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒可以做多少个样本?
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒分为2种规格,96孔和48孔。96孔的试剂盒,标曲和样本都做复孔的话,可以检测40个样本。96孔的试剂盒,标曲和样本都不做复孔的话,可以检测88个样本。
[2].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒使用视频?
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒实验操作视频在以下网址中,对每一步的实验步骤都做了演示,方便实验员能更好地理解ELISA实验的过程。
https://www.eiaab.com.cn/lesson-tech/805.html
https://www.eiaab.com.cn/lesson-tech/805.html
[3].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒是放在-20℃冰箱保存吗?
EIAab的人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒,洗涤液、底物、终止液保存于4℃,其余试剂-20℃冰箱保存。
[4].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒原理?
双抗体夹心法:用纯化的抗体包被微孔板,制成固相抗体,往包被有固相抗体的微孔中依次加入标准品或受检样本、生物素化抗体、HRP标记的亲和素,经过彻底洗涤后用底物TMB显色。用酶标仪在450nm波长下测定吸光度(OD值),计算样本浓度。
竞争法:用纯化的抗体包被微孔板,制成固相抗体,往包被有固相抗体的微孔中依次加入标准品或受检样本和生物素标记的目标分析物,受检标本中抗原与生物素标记抗原竞争结合有限的抗体。再加入HRP标记的亲和素,经过彻底洗涤后用底物TMB显色。用酶标仪在450nm波长下测定吸光度(OD值),计算样本浓度。
竞争法:用纯化的抗体包被微孔板,制成固相抗体,往包被有固相抗体的微孔中依次加入标准品或受检样本和生物素标记的目标分析物,受检标本中抗原与生物素标记抗原竞争结合有限的抗体。再加入HRP标记的亲和素,经过彻底洗涤后用底物TMB显色。用酶标仪在450nm波长下测定吸光度(OD值),计算样本浓度。
[5].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒中需要使用的样品量是多少?
夹心法100μL/孔,竞争法50μL/孔。如样本浓度过高时,应对样本进行稀释,以使稀释后的样本符合试剂盒的检测范围,计算时再乘以相应的稀释倍数。
[6].
如何分析人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒数据?
建议标准曲线,并计算样本浓度。对于elisa的曲线拟合,一般建议采用4参数曲线拟合,4参数曲线拟合通常更适合免疫分析。推荐使用专业软件进行曲线拟合,例如curve expert 1.3。根据样本的OD值由标曲查出相应的浓度,再乘以稀释倍数;或用标准物的浓度与OD值计算出标曲的回归方程式,将样本的OD值代入方程式,计算出样本浓度,再乘以稀释倍数,即为样本的实际浓度。以下链接是curve expert 1.3软件拟合曲线的方法。
https://www.eiaab.com.cn/news/502/
https://www.eiaab.com.cn/news/502/
[7].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒中是否包含人和动物的副产物,是否包含感染的或者传染性原料如HIV等?
除了抗体和稀释液中的BSA,不含其它人和动物的副产物,也不含感染材料。
[8].
收集人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒血浆样本,用什么作为抗凝剂?
一般建议用EDTA和肝素作为抗凝剂。
[9].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒酶标板可以拆成几部分?拆的时候是否需要避光,无菌?
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒酶标板是8×12孔条,可拆卸,板子可以拆成12条,注意避免孔污染,不需要避光和无菌。暂时不用的板子,放回原来装的袋子里,密封保存。
[10].
人胶原蛋白α -1(II)链的c端肽(CTXII)ELISA试剂盒样本如何保存?
尽量检测新鲜样本。若无新鲜样本,则4℃保存1周,-20℃保存1个月,-80℃保存2个月。
反馈墙
评论数 : 0
所有用户
所有用户
默认排序
默认排序
最近
早期
目前还没有评论。



通知
规格
数量
单价 (¥)
小计 1 (¥)
小计 2:
¥

规格
数量
单价 (¥)





 验证序列:
验证序列:




 折扣:
折扣: